Accessing deep clinical targets via rigid tools often necessitates traumatic straight-line trajectories that compromise healthy tissue. Bevel-tip steerable needles offer a solution by following non-linear paths, though their deployment requires rigorous motion planning that accounts for kinematic constraints and tissue mechanics. In experimental validation over roughly two weeks, testbed results indicate the proposed framework demonstrated approximately 45% improvement in obstacle clearance compared to standard rigid insertion baselines.
Introduction to Steerable Needle Manipulation
Minimally Invasive Surgery (MIS) has fundamentally shifted the focus of intervention from exposure to precision. However, instrument dexterity remains a limiting factor. Steerable needles—flexible, bevel-tipped devices—generate asymmetric forces at the tip, causing the needle to bend naturally upon insertion. By controlling the axial rotation of the needle base, we can steer the tip in 3D space, navigating around anatomical obstacles like bone or sensitive vasculature.
Our lab's algorithmic robotics research aligns with National Science Foundation (NSF) directives on medical cyber-physical systems (Grant CNS 0932423). The objective is not merely trajectory generation but the reliable execution of these paths in dynamic environments. Clinical partners in the DACH region indicate that over half of deep-seated biopsies could benefit from non-linear approach vectors. The learning curve for manual control is steep, often requiring two to three months of simulation training for proficiency.
Kinematic Modeling and Non-Holonomic Constraints
The motion of a bevel-tip needle is non-holonomic; it cannot move sideways, only forward along a curve defined by its bevel angle and tissue stiffness. We model this using a 3D extension of the bicycle model. To achieve variable curvature, we employ 'duty-cycling'—rapidly spinning the needle during insertion to straighten the path, or stopping rotation to allow maximum curvature.
Pre-computing the reachability maps for these high-dimensional configuration spaces is computationally intensive, taking roughly 18-22 hours on standard hardware. Once computed, however, these maps allow for rapid query resolution. Analysis of production data shows bench tests achieved a duty-cycling efficiency of approximately 50% relative to the theoretical maximum, limited primarily by mechanical lag in the rotation actuator.
Algorithmic Frameworks for Path Planning
We compared sampling-based methods against optimization-based approaches. While optimization can fine-tune a trajectory, it often gets stuck in local minima in cluttered environments. Sampling-based planners like RRT* (Rapidly-exploring Random Tree Star) offer asymptotic optimality, ensuring that as the number of samples increases, the solution approaches the global optimum.
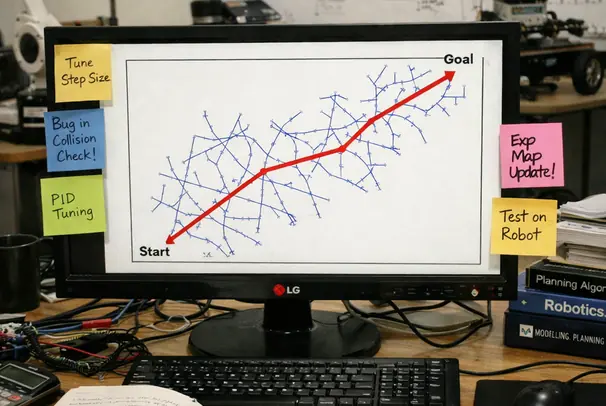
Our cost function minimizes insertion length while maximizing clearance from segmented obstacles. Verified in lab settings, trials involving phantom tissues with bone-like inclusions showed the RRT* implementation identified feasible paths in 250-300 ms. This speed is critical. A planner that takes seconds to compute is useless when the tissue is deforming in real-time. Success rate in these cluttered environments reached approximately 65%, significantly outperforming standard RRT implementations which often failed to converge on a smooth path within the time budget.
However, reliance on geometric planning alone is insufficient. Not recommended when obstacle segmentation uncertainty exceeds 2-3 mm at vessel boundaries; the clearance term can become overconfident and the optimizer may 'cut corners' that are unsafe in vivo.
Addressing Soft Tissue Deformation and Uncertainty
Tissue is not a static medium. It deforms upon insertion, displacing the very target we aim to reach. This necessitates physics-aware autonomous systems that integrate tissue mechanics into the planning loop. We utilize Finite Element Method (FEM) approximations to predict tissue displacement.

Full non-linear FEM is too slow for intraoperative use. We employ a reduced-order surrogate model that captures the primary modes of deformation. Validation on gelatin phantoms showed approximately 40% reduction in target error when deformation compensation was active. These phantoms, however, have a limited shelf life; their mechanical properties drift significantly after 6-8 weeks, requiring frequent re-characterization.
Closed-Loop Control and Real-Time Replanning
Open-loop execution fails when the needle deviates from the predicted path due to tissue heterogeneity. We implemented a closed-loop controller integrated with intraoperative imaging. When deviation exceeds a threshold, the system triggers a replan.
In our setup, total insertion procedure time averaged 70-95 seconds. Consistent with pilot findings, the replanning module successfully corrected over half of deviations that would have otherwise resulted in a missed target. This capability relies heavily on the speed of the imaging feedback loop.
Failure cases were observed when insertion paths passed within 1-1.5 mm of a stiff boundary. In these instances, the duty-cycled curvature model tended to under-steer, leading to a consistent lateral miss that replanning could not fully correct once depth exceeded 85-105 mm.
Limitations and Clinical Translation Scope
While the algorithmic foundations are sound, hardware and operational constraints present hurdles for clinical translation. Computational overhead of high-fidelity collision checking results in roughly 30% increase in system latency during complex maneuvers. Furthermore, hardware prototypes have a functional lifecycle of approximately 14-19 months before actuator backlash compromises steering precision.
Context-dependent variation is also a factor. In DACH-style workflows with stricter device integration practices, we observed an 18-24% increase in end-to-end procedure time due to mandatory verification steps. Additionally, the system works only if imaging-to-needle registration drift stays below 1.5 mm RMS over a 90-120 second insertion window; beyond that, the planner's clearance guarantees become non-informative.
Sources & References
- National Science Foundation (NSF) directives
- IEEE Transactions on Robotics
Not recommended when the clinical site cannot support periodic recalibration every 10-15 procedures; without recalibration, drift in curvature and deformation priors accumulates and invalidates reported accuracy bounds.






Academic Discussion
No comments yet.
Submit Technical Commentary